New Paper: Electrochemical Nitrate Conversion (ACS Catalysis)
Congrat to Dr. Jeonghoon Lim!! Examining bimetallic electrodes for stable conversion of nitrate to nitrogen gas.
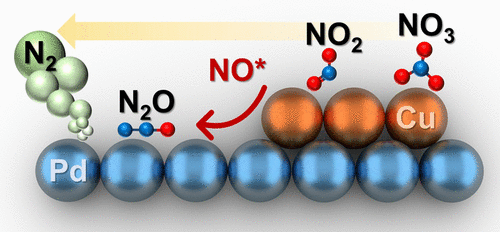
Electrocatalytic conversion of nitrate in waste can enable efficient waste remediation (NO3– to N2) or waste valorization (NO3– to NH4+) depending on the selectivity of the catalyst. Palladium and copper electrocatalysts typically exhibit ideal nitrate and nitrite binding properties, allowing for effective destruction of nitrate. However, rational steering of selectivity through material design remains a critical challenge for PdCu electrocatalyst. Here, we use the electrochemical underpotential deposition method to synthesize palladium nanocube electrocataysts with controlled copper surface coverage (e.g., partial and full copper coatings). We then examine the potential for NO3– destruction (conversion) and NO2– reduction reaction. We identify that partial copper-coated Pd nanocubes not only effectively facilitate the reduction of 95% of NO3– but also increase the reduction of NO2– to N2 with 89% selectivity over 20 consecutive cycles (80 h). We also show that under these conditions, the Pd(100) surface facet is exposed. Complete copper-covered Pd nanocubes effectively facilitate the reduction of ∼99% of NO3–. Complete coverage of copper; however, prevented exposure of Pd(100) surface facet, promoting the selective reduction of NO2– to NH4+ with a 70% selectivity over 20 consecutive cycles (80 h). Density functional theory (DFT) calculations show that NO3– and NO2– adsorb more strongly on the Cu(100) surface compared to the Pd(100) surface, while the NO* intermediate generated from NO3– or NO2– reduction adsorbs more strongly on the Pd surface. Barrier calculations show that NO* can readily migrate from the Cu domain to the Pd domain and that the N–N coupling barrier on Pd is significantly diminished at high NO* coverage. Together, these results suggest that the high N2 selectivity observed on the PdCu electrocatalyst is caused by the spillover of NO* from the Cu domains to the Pd domains.